Int J Drug Res Clin. 2023;1:e12.
doi: 10.34172/ijdrc.2023.e12
Original Article
The Effect of Short-term Methylprednisolone on Clinical Outcome and Lung CT Scan in Severe COVID-19: In-hospital and Six Weeks Later Follow-up
Maryam Sadat Mirenayat 1, 2  , Somaye Sadeghi 3, 4
, Somaye Sadeghi 3, 4  , Atefeh Fakharian 1, 2
, Atefeh Fakharian 1, 2  , Reyhaneh Zahiri 1, 2
, Reyhaneh Zahiri 1, 2  , Syed Bashir Mirtajani 5
, Syed Bashir Mirtajani 5  , Zahra Amraei 6
, Zahra Amraei 6  , Maryam Vasheghani 1, *
, Maryam Vasheghani 1, * 
Author information:
1Chronic Respiratory Diseases Research Center (CRDRC), National Research Institute of Tuberculosis and Lung Diseases (NRITLD), Shahid Beheshti University of Medical Sciences, Tehran, Iran
2Pulmonary Rehabilitation Research Center (PRRC), National Research Institute of Tuberculosis and Lung Disease (NRITLD), Shahid Beheshti University of Medical Sciences, Tehran, Iran
3Infectious Disease and Tropical Medicine Research Center, Isfahan University of Medical Sciences, Isfahan, Iran
4Acquired Immunodeficiency Research Center, Al-Zahra Hospital, Isfahan University of Medical Sciences, Isfahan, Iran
5Lung Transplantation Research Center, National Research Institute of Tuberculosis and Lung Diseases (NRITLD), Shahid Beheshti University of Medical Sciences, Tehran, Iran
6Faculty of Sport Sciences and Health, University of Shahid Beheshti, Tehran, Iran
Abstract
Background:
The severe coronavirus disease 2019 (COVID-19) induces widespread inflammatory responses that are attempted to be managed by the administration of anti-inflammatory drugs, along with antiviral drugs and respiratory support. This clinical trial study investigated the effect of short-term and low-dose methylprednisolone on the clinical outcome and radiological improvement of admitted patients with severe COVID-19.
Methods:
In this randomized clinical trial, two strains of patients with severe pneumonia of COVID-19 were evaluated before and after the use of corticosteroids. Inclusion criteria were age over 18 years, definitive case of COVID-19 based on positive reverse transcription polymerase chain reaction (RT-PCR) for severe acute respiratory syndrome coronavirus 2 (SARS-COV-2), and arterial oxygen saturation (SaO2)<93%; furthermore, at least 7 days and 5 days should be passed since the onset of symptoms and antiviral treatment, respectively. Exclusion criteria were similar to contraindications to the administration of methylprednisolone for short-term injections and pregnancy. Patients received intravenous methylprednisolone succinate (1-1.75 mg/kg/day) for 5 days in addition to treatment based on the World Health Organization (WHO) approved protocol for COVID-19. Finally, demographic characteristics, dyspnea, peripheral oxygen saturation (SpO2), number of lymphocytes, and computed tomography (CT) scan of the patient’s chest before and after taking corticosteroids were evaluated.
Results:
The average oxygen saturation of patients increased significantly after taking methylprednisolone succinate (P<0.001). Moreover, dyspnea and pulmonary insufficiency in CT scans improved significantly (P<0.001), and among hematological parameters, only lymphocytes increased significantly (P=0.01). Furthermore, methylprednisolone succinate use had no effect on mortality and length of hospitalization.
Conclusion:
Methylprednisolone succinate use during the treatment period of COVID-19 improves clinical conditions, CT scan findings, and hematological parameters affected by inflammation, but it does not affect the mortality and length of hospitalization of patients.
Keywords: SARS-COV-2, Severe COVID-19, Methylprednisolone, Inflammation
Introduction
The unprecedented health crisis caused by the coronavirus disease 2019 (COVID-19) has had various negative social and economic consequences and led to the death of countless people around the world. Obesity, male gender, diabetes, and genetic predisposition played a significant role in contracting COVID-19.1-3 After three years of the pandemic, new cases of COVID-19 continue to be reported in different parts of the world, and efforts to overcome the virus continue due to the lack of knowledge of the pathogen’s biology and the host’s responses to therapeutic pathways.
Based on the clinical manifestations and progression of the disease, three stages can be considered. The first or mild stage presents with mild symptoms (e.g., fever, cough, and sore throat) without pulmonary involvement and is treated symptomatically. The second stage, in the form of pneumonia, is accompanied by cough, fever, or hypoxia, and inflammatory markers increase slightly at this stage. At this stage, the patient is admitted to the hospital, and available antiviral treatments are applied. In the third stage, due to the severe systemic inflammatory response and cytokine storm, the patient develops acute respiratory distress syndrome (ARDS), severe hypoxia, multi-organ involvement, and eventually shock and death 3. In this stage, inflammatory factors such as C-reactive protein (CRP), D-dimer, interleukin-1, interleukin-2, and interleukin-6 increase strongly. At this stage, immunomodulatory and anti-inflammatory drugs such as corticosteroids can be effective. So far, no definitive treatment has been proposed for COVID-19, and an excessive inflammatory response can cause severe clinical symptoms and death.4
Many drug treatments such as low molecular weight heparin,5 hydroxychloroquine and azithromycin,6 lopinavir-ritonavir,7 remdesivir,8 and tocilizumab9 were used even though their convincing evidence was not available, and corticosteroids were no exception from this rule. At the beginning of the COVID-19 pandemic, the World Health Organization (WHO) did not approve the use of corticosteroids due to the lack of evidence of possible benefits and harm for severe acute respiratory syndrome (SARS).10,11 The use of steroids in patients with COVID-19 and ARDS has received attention after evidence of a reduction in patient mortality in China following the use of corticosteroids.12 Collecting evidence on the effectiveness of treatment with corticosteroids is very important because it can be beneficial to choose an effective treatment line when faced with other human coronaviruses. This study investigated the effect of methylprednisolone on clinical and radiologic improvement in patients with severe COVID-19 and its mortality.
Methods
The present study is a parallel double-blind clinical trial conducted at Dr. Masih Daneshvari hospital, a tertiary center for tuberculosis and respiratory disease in Tehran, Iran, from August 1, 2020 to November 30, 2020. Patients who were admitted and had persistent hypoxia due to severe pneumonia from COVID-19 were included in the study. Inclusion criteria were age 18 years and older, COVID-19 definitive case based on positive reverse transcription polymerase chain reaction (RT-PCR) for SARS coronavirus 2 (SARS-Cov-2), severe pneumonia according to WHO criteria, and persistent hypoxia (SaO2 < 93%) five days after the beginning of standard COVID-19 treatments according to the National Research Institute of Tuberculosis and Lung Disease (NRITLD) approved protocol.13 Exclusion criteria included hypersensitivity to methylprednisolone or any component of the formulation, systemic fungal infection, immune thrombocytopenia, herpes simplex of the eye, arrested tuberculosis, acute psychoses, Cushing syndrome, peptic ulcer, and pregnancy.
All patients underwent standard treatment provided by NRITLD based on international guidelines. Then patients were given 1-1.75 mg/kg/d intravenous methylprednisolone succinate (for 5 days). The primary outcomes were the level of dyspnea, oxygen saturation, blood lymphocyte count, and chest computed tomography (CT) scan findings. The secondary outcomes were the length of stay in the hospital and mortality. After obtaining written consent from patients, the patient’s history and clinical examination were obtained. Demographic characteristics, severity of dyspnea based on the visual analogue scale, vital signs, and arterial blood oxygen saturation (SaO2) were recorded. History and clinical examinations and follow-ups were performed by an internal assistant. Data were re-checked by two pulmonologists in charge of the ward. Laboratory tests including complete blood count with differential and lymphocyte counts were done before and after corticosteroid during hospitalization. Then, RT-PCR was performed for COVID-19 on the first day. Complete blood count with differential was done with a full automated auto analyzer (Automated Hematology Analyzer, Sysmex KX-21NTM, Japan), chest CT scan without contrast was performed on the first day and six weeks later. It scan was performed by a spiral multi slice (Siemens Co., Germany). Images were reviewed and reported separately by two radiologists.
Statistical Analysis
The sample size was calculated by the software of Cleveland University Library 9 based on the data in Young and colleagues’ study.14 The sample size for the two-sided unmatched clinical trial with type I error rate (α) = 0.05, power (1-β) = 0.80, case control ratio = 1:1, odds ratio = 0.059, probability of exposure in the control group (equivalence exposure) = 0.50, and Fleiss with correction for continuity was 41 in each group (total sample size = 82), as depicted in Figure 1. Patients were divided into case and control groups based on block randomization. The block size was 4, and 44 subjects were included in each group. Randomization was performed by a clinical pharmacologist in the hospital. The doctor and the patient did not know the grouping and the type of treatment.
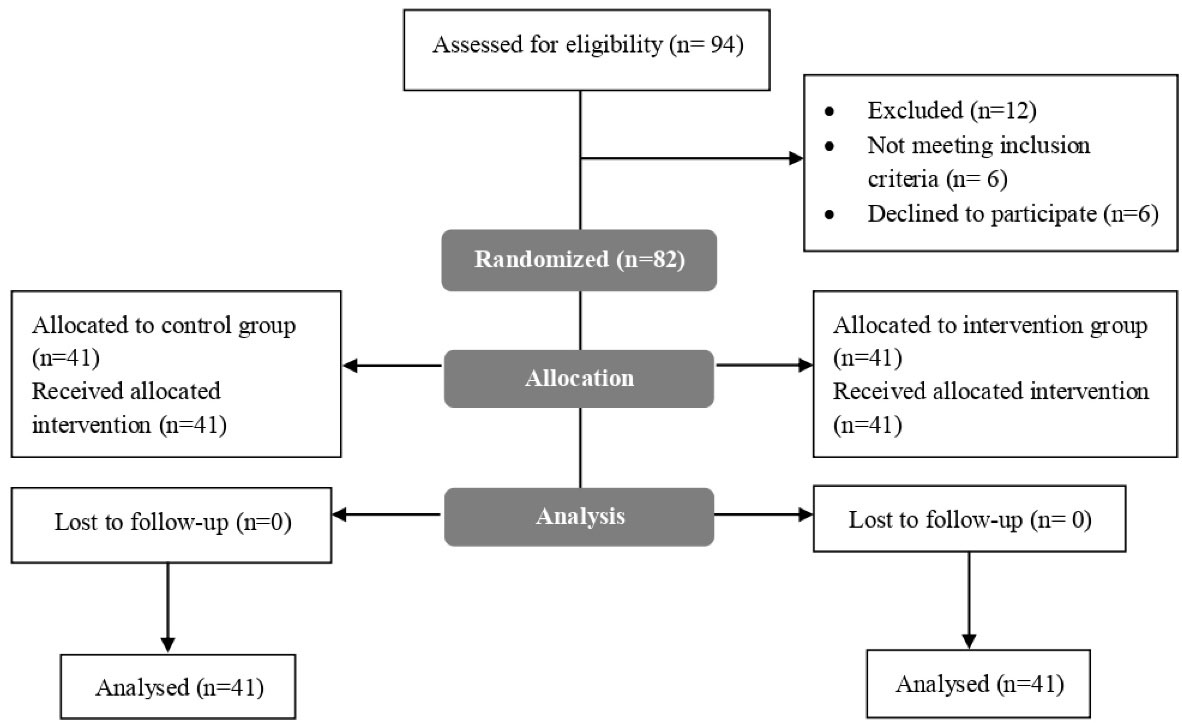
Figure 1.
Consort Flowchart of the Study
.
Consort Flowchart of the Study
After recording patient information, the data were analyzed by the SPSS software version 22. The frequency and percentage of qualitative and mean variables and the standard deviation of quantitative variables were calculated. Analytical statistics was used to compare study variables before and after the treatment. For comparing pre and post in one group, paired t-test was used, and repeated measure ANOVA was employed to analyze the data. Nonparametric Friedman and Mann-Whitney U tests were also used to compare the nonparametric data of dyspnea and CT scan.
Results
The present study was conducted on 86 hospitalized patients with COVID-19 with an average age of 16.04 ± 59.1 years, 39 of whom were women. The average length of hospitalization of the patients was 10.21 ± 14.37 days. Moreover, no mortality was reported in the patients participating in the study (Table 1).
Table 1.
Demographic Information of Patients Participating in the Study
|
Variable
|
Male (Mean±SD)
|
Female (Mean±SD)
|
Total (Mean±SD)
|
| Age |
39 (57.6 ± 16.6) |
47 (60.4 ± 14.6) |
86 (59.1 ± 16.04) |
| BMI |
26.04 ± 3.63 |
27.25 ± 6.82 |
26.71 ± 5.63 |
| Hospital stay |
14.46 ± 8.92 |
14.3 ± 11.26 |
14.37 ± 10.21 |
| Mortality |
0 |
0 |
0 |
Note. SD: Standard deviation; BMI: Body mass index.
The average SpO2 of patients before receiving methylprednisolone succinate was 83.22 ± 7.41, and after five days of use, it was 91.46 ± 5.03, showing that corticosteroid use significantly improved this variable (P < 0.001). Among the patients participating in the study, 47 (54.7%) patients had dyspnea, which decreased significantly to 13 (15.1%) after taking corticosteroids (P < 0.001).
Examining the CT results of the patients participating in the study revealed that 47 (100%) men manifested more than 50% lung involvement at the beginning of the study, which decreased to 23 (26.3%) after taking corticosteroids. Furthermore, among women, 39 (100%) of the total patients participating in the study exhibited more than 50% lung involvement, which decreased to 29 (35.5%) after taking corticosteroids. Accordingly, after taking a corticosteroid, a total of 52 patients (61.8%) exhibited lung involvement of higher than 50% in CT.
The examination of patients with less than 50% involvement in CT indicated that there are no patients with less than 50% lung involvement at the beginning of the study. After taking corticosteroids, 18 men (19.7%) recovered, and the number of woman patients with pulmonary involvement of less than 50% reached 16 (18.4%) patients. Therefore, of the 86 patients participating in the current study, 34 exhibited pulmonary involvement under 50% in their CT after taking corticosteroids. In total, the number of patients with pulmonary involvement caused by COVID-19 was associated with a significant decrease after taking corticosteroids (P < 0.001).
Comparing the mean of the patient’s white blood cells before taking corticosteroids (3.67 ± 7.3) and after taking this drug (8.2 ± 3.6) did not display any significant difference (P = 0.34). The mean of lymphocytes of the patients suffering from COVID-19 participating in this study was 9.7 ± 19.59 at the beginning and reached 24.54 ± 12.97 after corticosteroid use, indicating a significant increase in lymphocytes due to corticosteroid use (P = 0.01). Based on the obtained results, there was no significant difference in lactate dehydrogenase (LDH) levels of patients after taking corticosteroids (P = 0.7).
Moreover, the mean of patients’ fast blood sugar at the beginning was 128.31 ± 64.73 and reached 145.32 ± 54.2 after taking methylprednisolone succinate, suggesting no significant difference (P = 0.18). Additionally, there was no significant difference in the average vitamin D changes of patients (P = 0.2) after taking corticosteroids (before treatment 7.26 ± 18.25 and after treatment 6.9 ± 17.5), as illustrated in Table 2.
Table 2.
The Comparison of Hematological Factors and Clinical Symptoms of Patients before and After Taking Methylprednisolone Succinate
|
Variable
|
Groups
|
P
Value
|
Total (N=86)
|
P
Value
|
|
Male (n=47)
|
Female (n=39)
|
|
Pre
|
Post
|
Pre
|
Post
|
Pre
|
Post
|
| SpO2 (%) |
82.82 ± 6.22 |
91.5 ± 5.57 |
83.55 ± 8.33 |
91.4 ± 4.04 |
0.36 |
83.22 ± 7.41 |
91.46 ± 5.03 |
< 0.001 |
| Dyspnea |
Yes |
26 (30.2%) |
6 (7%) |
21 (24.4%) |
7 (8.1%) |
0.95 |
47 (54.7%) |
13 (15.1%) |
< 0.001 |
| No |
13 (15.1%) |
33 (46.5%) |
26 (38.4%) |
39 (46.5%) |
39 (45.3%) |
73 (84.9%) |
| CT (%) |
> 50% |
47 (100%) |
23 (26.3%) |
39 (100%) |
29 (35.5%) |
0.53 |
86 (100%) |
52 (61.8%) |
< 0.001 |
| < 50% |
0 |
18 (19.7%) |
0 |
16 (18.4%) |
0 |
34 (38.2%) |
| WBC (n/mm3) |
8.33 ± 4.55 |
8.36 ± 3.83 |
6.6 ± 2.7 |
8.08 ± 3.4 |
0.16 |
7.3 ± 3.67 |
8.2 ± 3.6 |
0.34 |
| LYMPH (103/µL) |
16.89 ± 8.37 |
26.92 ± 16.19 |
21.34 ± 9.67 |
22.83 ± 9.99 |
0.01 |
19.59 ± 9.7 |
24.54 ± 12.97 |
0.01 |
| LDH (mg/dL) |
525.73 ± 185.89 |
492.43 ± 124.29 |
530.49 ± 175.99 |
503.67 ± 150.29 |
0.63 |
528.58 ± 178.59 |
495.8 ± 128.61 |
0.7 |
| FBS (mg/dL) |
121.14 ± 31.84 |
139.93 ± 62.38 |
135 ± 84.28 |
152.18 ± 43.59 |
0.57 |
128.31 ± 64.73 |
145.32 ± 54.2 |
0.18 |
| Vitamin D (ng/mL) |
19.36 ± 6.45 |
19.48 ± 7.33 |
17.1 ± 5.5 |
16.9 ± 4.98 |
.5 |
18.25 ± 7.26 |
17.5 ± 6.9 |
.2 |
Note. SpO2: Peripheral oxygen saturation; CT: Computed tomography; WBC: White blood cell; LDH: Lactate dehydrogenase; LYMPH: Lymphocyte; FBS: Fast blood sugar.
In addition, the results obtained from the independent t-test of the investigated variables in Table 2, taking into account the effect of gender, indicated that among the investigated variables, only the number of lymphocytes indicated a significant change due to the use of corticosteroids (P = 0.01).
Discussion
At the beginning of the COVID-19 pandemic, corticosteroids were prescribed cautiously. After the first studies that were published regarding reducing the possibility of mortality with the use of corticosteroids in ARDS caused by COVID-19, the use of these drugs received greater attention.15,16 However, different studies came up with different results.
In the present study, treatment with corticosteroids did not effect on the mortality and length of hospitalization of patients with COVID-19 as a confounding factor. Previous studies indicated that early use of dexamethasone can reduce the duration of mechanical ventilation and mortality in patients with moderate to severe ARDS by affecting the immune response.17 Furthermore, a clinical trial entitled RECOVERY trial showed that the use of corticosteroids reduces mortality by 3% in COVID-19 patients.18 However, the results obtained from some studies make the use of corticosteroids questionable. A clinical trial revealed that the delayed use of corticosteroids has a positive effect on ARDS and increases mortality.19 In addition, reports of increased weakness due to long hospitalization in the intensive care unit (ICU) are available20 that suggest an increase in infection,21 hypernatremia,22 and hyperglycemia.23
Based on our results, the oxygen saturation of patients treated with methylprednisolone succinate revealed a significant increase. In addition, the use of this drug led to the improvement of dyspnea in the patients. The findings of the CT scan of the patients also showed that the pulmonary involvement of the patients improves significantly after the course of corticosteroid use.
We hope that the results of this study, together with other studies that have been conducted, can help provide a better understanding of the effect of corticosteroids in the treatment of COVID-19. Furthermore, randomized clinical trials should always be compared with data from similar studies to help reach more reliable results because many studies lose significance over time.
A positive outcome of corticosteroid treatment was the improvement of factors involved in inflammation. Previous studies demonstrated that patients with COVID-19 who need hospitalization in ICU show an increase in leukocytes and neutrophils, a decrease in lymphocytes, an increase in LDH, and an increase in platelets.24,25 In the present study, it was found that the use of corticosteroids can reduce the probability of hospitalization of patients in the ICU by increasing lymphocytes and white blood cells and also decreasing lactate dehydrogenase. It was previously confirmed that the increase in LDH during admission to the hospital may be a predictor of the final admission to the ICU.26
Despite the results we obtained, the effect of corticosteroid dosage should not be ignored. In our patients, the dose used was 1-1.75 mg/kg/d intravenous methylprednisolone succinate (for 5 days). Recent data have revealed that a dose lower than 1 mg/kg/d of prednisone (equivalent to 0.15 mg per day of dexamethasone) has no effect on the mortality of patients.27 On the other hand, the use of higher doses can increase the risk of mortality in severe COVID-19 patients.28
Limitations
Due to the cross-sectional and single-center nature of this study, the number of available data was limited, and the results of this study cannot be used alone to determine the effectiveness of low-dose corticosteroids in all COVID-19 patients. It seems that reasonable results can be obtained by considering other intervening variables under clinical conditions and examining effective factors in immune responses with the help of data obtained from several treatment centers and comparing them with each other.
Conclusion
Treatment with corticosteroids did not lead to an increase in mortality and length of hospitalization in patients with COVID-19. The benefit of using corticosteroids has been an improvement in clinical symptoms, respiratory failure due to lung injury, oxygen saturation, and hematological factors increased by inflammation. In addition, meta-analyses using data from this study and other similar observational studies can provide more reliable results.
Ethics statement
The study protocol was approved by the Research Ethics Committee of Shahid Beheshti University, Tehran, Iran, with code IR.SBMU.NRITLD.REC.1399.074 and registered in the Clinical Trial Center of Iran with code IRCT20200611047727N3.
Disclosure of funding source
There was no financial support for the present study.
Conflict of interests declaration
None to declare.
Acknowledgments
We recognize the worth of all the colleagues who passed away during the fight against COVID-19, and we are grateful to all people who contributed to the preparation of this study.
Data availability statement
Supporting data for this study are available and will be provided to the applicant upon reasonable request from the corresponding author.
Consent for publication
Not applicable.
References
- Amirsavadkouhi A, Jahangirifard A, Shahrami R, Safari S, Feizabadi F, Mirshafiei Z. The role of hemoperfusion in COVID-19 infection: a case series. Arch Anesth Crit Care 2021; 7(3):189-194. doi: 10.18502/aacc.v7i3.6912 [Crossref] [ Google Scholar]
- Fakharian A, Barati S, Mohamadi M, Dastan F. Successful management of COVID-19 with adalimumab in a post-coronary artery bypass graft surgery patient. J Cardiothorac Vasc Anesth 2022; 36(4):1115-7. doi: 10.1053/j.jvca.2020.12.023 [Crossref] [ Google Scholar]
- Tahsini Tekantapeh S, Mikaeili H, Soleimanpour H. The role of respiratory system surface area and ventilation volume in severity and mortality of COVID-19 infection. Front Emerg Med 2021; 5(3):e27. doi: 10.18502/fem.v5i3.5889 [Crossref] [ Google Scholar]
- Pascarella G, Strumia A, Piliego C, Bruno F, Del Buono R, Costa F. COVID-19 diagnosis and management: a comprehensive review. J Intern Med 2020; 288(2):192-206. doi: 10.1111/joim.13091 [Crossref] [ Google Scholar]
- Zhang W, Zhao Y, Zhang F, Wang Q, Li T, Liu Z. The use of anti-inflammatory drugs in the treatment of people with severe coronavirus disease 2019 (COVID-19): the perspectives of clinical immunologists from China. Clin Immunol 2020; 214:108393. doi: 10.1016/j.clim.2020.108393 [Crossref] [ Google Scholar]
- Hippensteel JA, LaRiviere WB, Colbert JF, Langouët-Astrié CJ, Schmidt EP. Heparin as a therapy for COVID-19: current evidence and future possibilities. Am J Physiol Lung Cell Mol Physiol 2020; 319(2):L211-l7. doi: 10.1152/ajplung.00199.2020 [Crossref] [ Google Scholar]
- Gautret P, Lagier JC, Parola P, Hoang VT, Meddeb L, Mailhe M. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int J Antimicrob Agents 2020; 56(1):105949. doi: 10.1016/j.ijantimicag.2020.105949 [Crossref] [ Google Scholar]
- Verdugo-Paiva F, Izcovich A, Ragusa M, Rada G. Lopinavir-ritonavir for COVID-19: a living systematic review. Medwave 2020; 20(6):e7967. doi: 10.5867/medwave.2020.06.7966 [Crossref] [ Google Scholar]
- Beigel JH, Tomashek KM, Dodd LE, Mehta AK, Zingman BS, Kalil AC. Remdesivir for the treatment of COVID-19 - final report. N Engl J Med 2020; 383(19):1813-26. doi: 10.1056/NEJMoa2007764 [Crossref] [ Google Scholar]
- Lan SH, Lai CC, Huang HT, Chang SP, Lu LC, Hsueh PR. Tocilizumab for severe COVID-19: a systematic review and meta-analysis. Int J Antimicrob Agents 2020; 56(3):106103. doi: 10.1016/j.ijantimicag.2020.106103 [Crossref] [ Google Scholar]
- Arabi YM, Mandourah Y, Al-Hameed F, Sindi AA, Almekhlafi GA, Hussein MA. Corticosteroid therapy for critically ill patients with Middle East respiratory syndrome. Am J Respir Crit Care Med 2018; 197(6):757-67. doi: 10.1164/rccm.201706-1172OC [Crossref] [ Google Scholar]
- World Health Organization. Clinical management of severe acute respiratory infection (SARI) when COVID-19 disease is suspected Interim guidance. Pediatr Med Rodz 2020; 16(1):9-26. doi: 10.15557/PiMR.2020.0003 [Crossref] [ Google Scholar]
- Mirenayat MS, Abedini A, Kiani A, Eslaminejad A, Adimi Naghan P, Malekmohammad M. National Research Institute of Tuberculosis and Lung Disease (NRITLD) protocol for the treatment of patients with COVID-19. Iran J Pharm Res 2022; 21(1):e123947. doi: 10.5812/ijpr.123947 [Crossref] [ Google Scholar]
- Yang R, Xiong Y, Ke H, Chen T, Gao S. The role of methylprednisolone on preventing disease progression for hospitalized patients with severe COVID-19. Eur J Clin Invest 2020; 50(11):e13412. doi: 10.1111/eci.13412 [Crossref] [ Google Scholar]
- Alhazzani W, Evans L, Alshamsi F, Møller MH, Ostermann M, Prescott HC. Surviving sepsis campaign guidelines on the management of adults with coronavirus disease 2019 (COVID-19) in the ICU: first update. Crit Care Med 2021; 49(3):e219-e34. doi: 10.1097/ccm.0000000000004899 [Crossref] [ Google Scholar]
- Rezk NA, Ibrahim AM. Effects of methyl prednisolone in early ARDS. Egypt J Chest Dis Tuberc 2013; 62(1):167-72. doi: 10.1016/j.ejcdt.2013.02.013 [Crossref] [ Google Scholar]
- Fakharian A, Mirenayat MS, Ferdowsi F, Mirtajani SB, Khalili V, Zahiri R. The efficacy of methylprednisolone in clinical manifestations, inflammatory biomarkers, and antioxidant changes in the COVID-19 patients. Arch Clin Infect Dis 2022; 17(4):e129799. doi: 10.5812/archcid-129799 [Crossref] [ Google Scholar]
- Villar J, Ferrando C, Martínez D, Ambrós A, Muñoz T, Soler JA. Dexamethasone treatment for the acute respiratory distress syndrome: a multicentre, randomised controlled trial. Lancet Respir Med 2020; 8(3):267-76. doi: 10.1016/s2213-2600(19)30417-5 [Crossref] [ Google Scholar]
- Xu J, Yang X, Yang L, Zou X, Wang Y, Wu Y. Clinical course and predictors of 60-day mortality in 239 critically ill patients with COVID-19: a multicenter retrospective study from Wuhan, China. Crit Care 2020; 24(1):394. doi: 10.1186/s13054-020-03098-9 [Crossref] [ Google Scholar]
- Steinberg KP, Hudson LD, Goodman RB, Hough CL, Lanken PN, Hyzy R. Efficacy and safety of corticosteroids for persistent acute respiratory distress syndrome. N Engl J Med 2006; 354(16):1671-84. doi: 10.1056/NEJMoa051693 [Crossref] [ Google Scholar]
- Yang T, Li Z, Jiang L, Xi X. Corticosteroid use and intensive care unit-acquired weakness: a systematic review and meta-analysis. Crit Care 2018; 22(1):187. doi: 10.1186/s13054-018-2111-0 [Crossref] [ Google Scholar]
- Giacobbe DR, Battaglini D, Ball L, Brunetti I, Bruzzone B, Codda G. Bloodstream infections in critically ill patients with COVID-19. Eur J Clin Invest 2020; 50(10):e13319. doi: 10.1111/eci.13319 [Crossref] [ Google Scholar]
- Fang F, Zhang Y, Tang J, Lunsford LD, Li T, Tang R. Association of corticosteroid treatment with outcomes in adult patients with sepsis: a systematic review and meta-analysis. JAMA Intern Med 2019; 179(2):213-23. doi: 10.1001/jamainternmed.2018.5849 [Crossref] [ Google Scholar]
- Meijvis SC, Hardeman H, Remmelts HH, Heijligenberg R, Rijkers GT, van Velzen-Blad H. Dexamethasone and length of hospital stay in patients with community-acquired pneumonia: a randomised, double-blind, placebo-controlled trial. Lancet 2011; 377(9782):2023-30. doi: 10.1016/s0140-6736(11)60607-7 [Crossref] [ Google Scholar]
- Regolo M, Vaccaro M, Sorce A, Stancanelli B, Colaci M, Natoli G. Neutrophil-to-lymphocyte ratio (NLR) is a promising predictor of mortality and admission to intensive care unit of COVID-19 patients. J Clin Med 2022; 11(8):2235. doi: 10.3390/jcm11082235 [Crossref] [ Google Scholar]
- Seyit M, Avci E, Nar R, Senol H, Yilmaz A, Ozen M. Neutrophil to lymphocyte ratio, lymphocyte to monocyte ratio and platelet to lymphocyte ratio to predict the severity of COVID-19. Am J Emerg Med 2021; 40:110-4. doi: 10.1016/j.ajem.2020.11.058 [Crossref] [ Google Scholar]
- Wu MY, Yao L, Wang Y, Zhu XY, Wang XF, Tang PJ. Clinical evaluation of potential usefulness of serum lactate dehydrogenase (LDH) in 2019 novel coronavirus (COVID-19) pneumonia. Respir Res 2020; 21(1):171. doi: 10.1186/s12931-020-01427-8 [Crossref] [ Google Scholar]
- Schimmer BP, Funder JW. ACTH, adrenal steroids, and pharmacology of the adrenal cortex. In: Goodman and Gilman’s The Pharmacological Basis of Therapeutics. 12th ed. New York: McGraw Hill; 2011. p. 1209-35.