Int J Drug Res Clin. 2023;1:e20.
doi: 10.34172/ijdrc.2023.e20
Original Article
Assessment of the Effect of Elaeagnus angustifolia on Anthropometric Characteristics and Dietary Intake in Patients with Osteoarthritis: A Randomized Clinical Trial
Zeinab Nikniaz 1, *  , Reza Mahdavi 2, Leila Nikniaz 3
, Reza Mahdavi 2, Leila Nikniaz 3
Author information:
1Liver and Gastrointestinal Diseases Research Center, Tabriz University of Medical Sciences, Tabriz, Iran
2Nutrition Faculty, Tabriz University of Medical Sciences, Tabriz, Iran
3Tabriz Health Services Management Research Center, Tabriz University of Medical Sciences, Tabriz, Iran
Abstract
Background:
Considering the result of animal studies regarding the effect of Elaeagnus angustifolia (EA) on weight, the present study was designed to assess the effect of whole fruit and medulla of EA powder supplementation on weight and dietary intake of patients with knee osteoarthritis (OA).
Methods:
In this interventional study, 90 female patients with OA were allocated into whole fruit powder of EA (15 g/d), medulla powder of EA (15 g/d), and placebo groups. The supplementation duration was eight weeks. The weight and height were measured using the standard protocol, and the body mass index (BMI) was calculated. The dietary intake was assessed using the 24-hour recall for three days and analyzed using Nutritionist IV software. Then, paired t-tests and analysis of variance (ANOVA) were used as a statistical analysis.
Results:
A total of 78 patients participated in this study. The mean age of participants was 56.31±8.55 years, and the mean BMI was 32.44±2.95 kg/m2. None of the participants were physically active, 69% of them were inactive, and 31% were minimally active. There were no significant differences between groups regarding the baseline values. The mean BMI decreased in the EA medulla and whole fruit group, and it increased in the placebo group. However, the changes were not significant in the studied groups (P>0.05). The results of within-group analyses indicated no significant changes in energy, macronutrients, and antioxidant micronutrient intake after EA medulla, whole fruit, and placebo supplementation compared with baseline values (P>0.05). The result of between-group analyses also showed no significant difference between groups regarding the changes in energy, macro, and micronutrient intake.
Conclusion:
The results of the present clinical trial indicated that EA has no significant effect on anthropometric characteristics and dietary intake in patients with OA.
Keywords: Elaeagnus angustifolia, Body mass index, Anthropometric measures, Osteoarthritis, Dietary intake
Copyright and License Information
© 2023 The Author(s).
This is an open-access article distributed under the terms of the Creative Commons Attribution License (
https://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Knee osteoarthritis (OA) is one of the predominant musculoskeletal diseases1 with a high burden of disease,2 and it is the most prevalent in females.3 Different causes including age, gender, obesity, and knee injury were considered as risk factors of OA.1 Obesity is one of the main reasons for the development and progression of knee OA due to both increased inflammation state and mechanical stress. There is a dose-response association between obesity and the risk of knee OA, and this association is significantly stronger for females than for males.4,5 It has been indicated that weight reduction reduced inflammation and pain and amended function in patients with knee OA.4,5
Different treatments are incorporated into the management protocol of OA to decrease pain and inflammation and preserve the function of joints.6 However, these treatments have partial effectiveness and are related to side effects.7 Therefore, nowadays, nonpharmacological approaches are recommended along with pharmacological approaches for the treatment of OA.8 Herbal medicine is one of the non-pharmacological interventions that is recommended for the treatment of OA.8 The effect of different herbs, including green tea9 and Zingiber10 have been investigated in OA management.
Elaeagnus angustifolia (EA) is another herb with anti-inflammatory effects11 that has been studied in the treatment of respiratory, gastrointestinal diseases, and kidney disorders.12,13 Moreover, it is used for pain relief in rheumatoid arthritis.12 Previously, it was demonstrated that this fruit has anti-inflammatory,11 antioxidant,14 and serum lipid-lowering effects15 in patients with OA. Moreover, animal studies showed that the administration of 250 and 500 mg/kg aqueous extract of EA to rats can decrease weight and dietary intake in a dose-response manner.16
To the best of our knowledge, there is no human study on the effect of EA on weight. Considering the effect of obesity on OA progression and owing to the result of animal studies regarding the effect of EA on weight, the present study was designed to assess the effect of whole fruit and medulla of EA L. powders supplementation on weight and dietary intake of patients with knee OA.
Methods
In the present clinical trial, we selected 90 patients with OA. The inclusion criteria were obese (Body mass index [BMI] of 30-34.9 kg/m2) female patients aged 40-70 years and patients with mild to moderate severity of knee OA (according to the American College of Rheumatology classification). The patients excluded from the study had secondary OA, synovial inflammation, movement disorders, active hypertension, heart disease, renal disease, and impairment of liver, used diuretic drugs such as furosemide, uricosurics such as probenecid, anticoagulants, anticonvulsant, sulfa drugs, methotrexate, psychiatric medication such as lithium, beta-blockers, and muscle relaxants, were smokers, showed hypersensitivity responses to EA, and used EA frequently.
Study Design
In the present study, 90 participants were randomly allocated into the two interventions (medulla, and whole fruit groups) and a placebo group. The random list was generated using the GraphPad by a researcher who was not involved in other part of the research, and the list was kept in an opaque envelope. Thirty patients were included in each group. The patients in the intervention groups received 15 mg/d of whole EA powder or EA medulla powder, while the patients in the placebo group received 15 mg/d of corn starch. The supplementation duration in all groups was 8 weeks, and the patients and the outcome assessor were blind. Furthermore, the conventional physician visits and treatments were constant during the investigation period.
Elaeagnus angustifolia Preparation
All EA were purchased from one center in Tabriz in December and identified in the Pharmacognosy laboratory of Tabriz University of medical sciences (Voucher Number: TBZ-fph 721). One part of the samples was turned into the whole powder by the Piazar company. The medulla powder was prepared by a grater after peeling the skin of EA. Then, all samples were packed in 15 g bags in the same opaque nylons.
Outcome
Assessing the effect of EA supplementation on dietary intake and anthropometric measurement was the secondary objective of the study. The result of the primary goal of this investigation was previously published.11
Anthropometric measurements in the present study were weight, height, and BMI. Standing height was measured by a stadiometer. The patients were asked to relax their arms, look straight, breathe normally, get their knees and heels together, and wear no shoes and hair ties. The height was documented to the nearest 0.1 cm. Weight was measured in light indoor clothing with shoes removed with a Seca scale to the nearest 0.5 kilogram. The BMI was calculated based on the weight in kilograms (or pounds) divided by the square of height in meters.
Dietary intake was assessed using three days of 24-hour food recall before and just after intervention. The Iranian-modified Nutritionist IV software was used for the determination of energy, macro, and micronutrient intake.
For the determination of physical activity level, the International Physical Activity Questionnaire (IPAQ) was used. For this, the type of physical activity, duration, and number of days in weeks the physical activity was done were recorded. Then, the metabolic equivalent of task (METs) was calculated. The patients were divided into three groups based on METs/min/wk: low active ( < 600 METs/min/wk), moderately active (600 < METs/min/wk < 3000), and vigorously active ( > 3000 METs/min/wk).17
Statistical Analysis
We analyzed the data using SPSS version 16. For determination of the data distribution normality, the Kolmogorov-Smirnov test was applied, and the paired sample t-test was applied to compare the before/after results. In addition, the between-group comparisons were done by one-way analysis of variance (ANOVA), and the significance level was set at 5%.
Results
In the present clinical trial, 90 patients were randomly allocated to three groups. Four patients in the whole fruit powder group, three patients in the medulla group, and 5 patients in the placebo group were lost to follow-up (Figure 1). The mean age of participants was 56.31 ± 8.55 years, and the mean BMI was 32.44 ± 2.95 kg/m2. The mean baseline calorie, carbohydrate, protein, and fat intake were 2006 ± 413 kcal/day, 266.90 ± 49.20 g/d, 65.49 ± 15.00 g/d, and 66.59 ± 23.53 g/d, respectively (Table 1). As shown in Figure 2, none of the participants were physically active, 69% of them were inactive, and 31% of them were minimally active. Furthermore, there were no significant differences between groups regarding the baseline values and physical activity level (Figure 2).
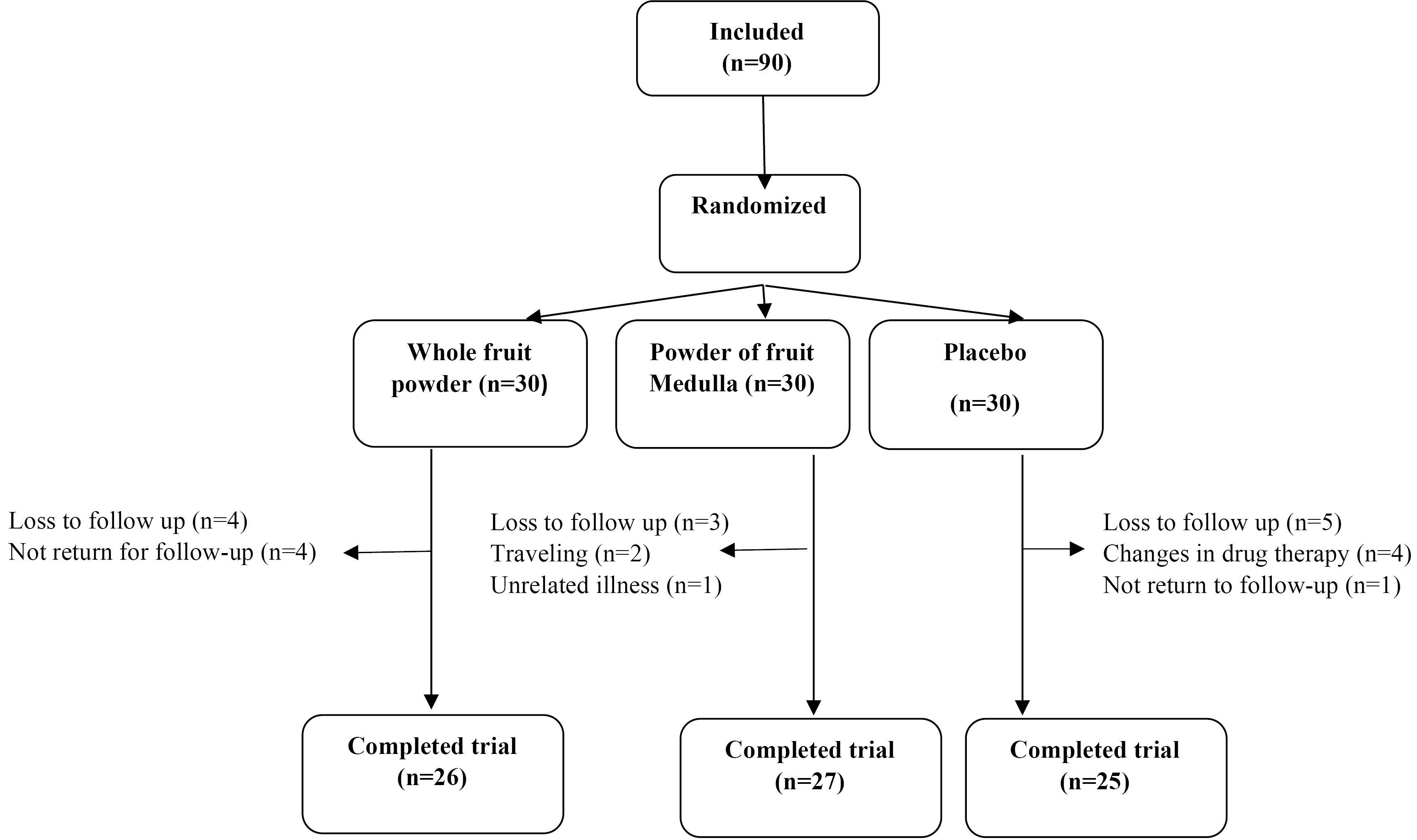
Figure 1.
Flow Chart for Patient Enrolment, Randomization, and Retention
.
Flow Chart for Patient Enrolment, Randomization, and Retention
Table 1.
Comparison of the Mean Fietary Intake Between Groups
|
Variables
|
Medulla (n=27)
|
Whole Fruit (n=26)
|
Placebo (n=25)
|
P
value
|
|
Before Intervention
|
After Intervention
|
P
Value
|
Before Intervention
|
After Intervention
|
P
Value
|
Before Intervention
|
After Intervention
|
P
Value
|
| Energy (kcal/d) |
2045 ± 441 |
1852 ± 430 |
0.41 |
1935 ± 315 |
1828 ± 440 |
0.33 |
1989 ± 467 |
2056 ± 609 |
0.50 |
0.30 |
| Carbohydrates (g/d) |
291.52 ± 43.6 |
254.08 ± 70.15 |
0.36 |
259.5 ± 61.39 |
246 ± 65.54 |
0.52 |
260.91 ± 36.91 |
266.22 ± 86.91 |
0.87 |
0.20 |
| % Energy from carbohydrates |
57.70 ± 14.5 |
54.35 ± 14.21 |
0.41 |
64.01 ± 13.11 |
59.91 ± 15.81 |
0.17 |
52.53 ± 12.25 |
52.7 ± 11.12 |
0.20 |
0.30 |
| Protein (g/d) |
66.28 ± 15.2 |
55.84 ± 16.54 |
0.51 |
13.36 ± 3.4 |
13.20 ± 2.1 |
0.31 |
69.10 ± 16.86 |
63.03 ± 17.09 |
0.85 |
0.40 |
| % Energy from protein |
12.96 ± 3.1 |
12.06 ± 3.7 |
0.22 |
67.81 ± 21.43 |
62.80 ± 22.87 |
0.43 |
13.86 ± 3.11 |
12.6 ± 4.1 |
0.43 |
0.60 |
| Fat (g/d) |
67.2 ± 16.18 |
66.91 ± 21.16 |
0.39 |
67.81 ± 21.43 |
62.80 ± 22.87 |
0.43 |
69.50 ± 31.15 |
73.69 ± 25.14 |
0.79 |
0.7 |
| % Energy from fat |
29.18 ± 5.6 |
30.30 ± 4.1 |
0.30 |
31.21 ± 5.2 |
30.95 ± 4.3 |
0.20 |
31.96 ± 5.7 |
33.42 ± 4.8 |
0.4 |
0.4 |
| PUFA (g/d) |
12.44 ± 5.12 |
12.84 ± 3.34 |
0.31 |
13.87 ± 2.25 |
15.51 ± 5.75 |
0.31 |
14.58 ± 3.45 |
14.67 ± 5.34 |
0.51 |
0.53 |
| MUFA (g/d) |
21.52 ± 3.34 |
21.95 ± 3.45 |
0.33 |
23.49 ± 5.33 |
24.92 ± 2.75 |
0.32 |
24.1 ± 4.34 |
23.67 ± 2.46 |
0.31 |
0.64 |
| SFA (g/d) |
13.30 ± 4.64 |
13.09 ± 3.23 |
0.32 |
14.28 ± 3.45 |
14.22 ± 3.64 |
0.33 |
14.82 ± 3.64 |
13.20 ± 4.34 |
0.32 |
0.43 |
| Fiber (g/d) |
15.54 ± 5.56 |
15.89 ± 3.12 |
0.42 |
16.21 ± 6.01 |
16.78 ± 5.84 |
0.45 |
16.57 ± 6.17 |
16.35 ± 6.37 |
0.43 |
0.63 |
| Vitamin A (µg/d) |
750.6 ± 103 |
682.3 ± 101 |
0.15 |
1003 ± 208.5 |
1166.2 ± 247.6 |
0.77 |
599.72 ± 50.2 |
61.5 ± 97.6 |
0.26 |
0.60 |
| Vitamin E (mg/d) |
7.39 ± 2.43 |
6.64 ± 0.28 |
0.64 |
7.04 ± 0.35 |
6.67 ± 0.3 |
0.29 |
7.01 ± 1.31 |
6.67 ± 0.3 |
0.68 |
0.72 |
| Vitamin C (mg/d) |
67.80 ± 54.6 |
35.5 ± 6.4 |
0.58 |
40.96 ± 38.5 |
49.05 ± 11.6 |
0.52 |
37.66 ± 32.8 |
49.71 ± 10.1 |
0.20 |
0.15 |
| Zinc (mg/d) |
6.51 ± 4.5 |
9.7 ± 4.2 |
0.51 |
4.98 ± 3.2 |
4.87 ± 1.34 |
0.89 |
5.9 ± 7.3 |
7.18 ± 2.3 |
0.68 |
0.70 |
| Selenium (µg/d) |
0.06 ± 0.02 |
0.05 ± 0.03 |
0.58 |
0.05 ± 0.003 |
0.08 ± 0.001 |
0.14 |
0.08 ± 0.001 |
0.09 ± 0.007 |
0.20 |
0.30 |
Note. PUFA: Polyunsaturated fatty acid; MUFA: Monounsaturated fatty acid; SFA: Saturated fatty acid.
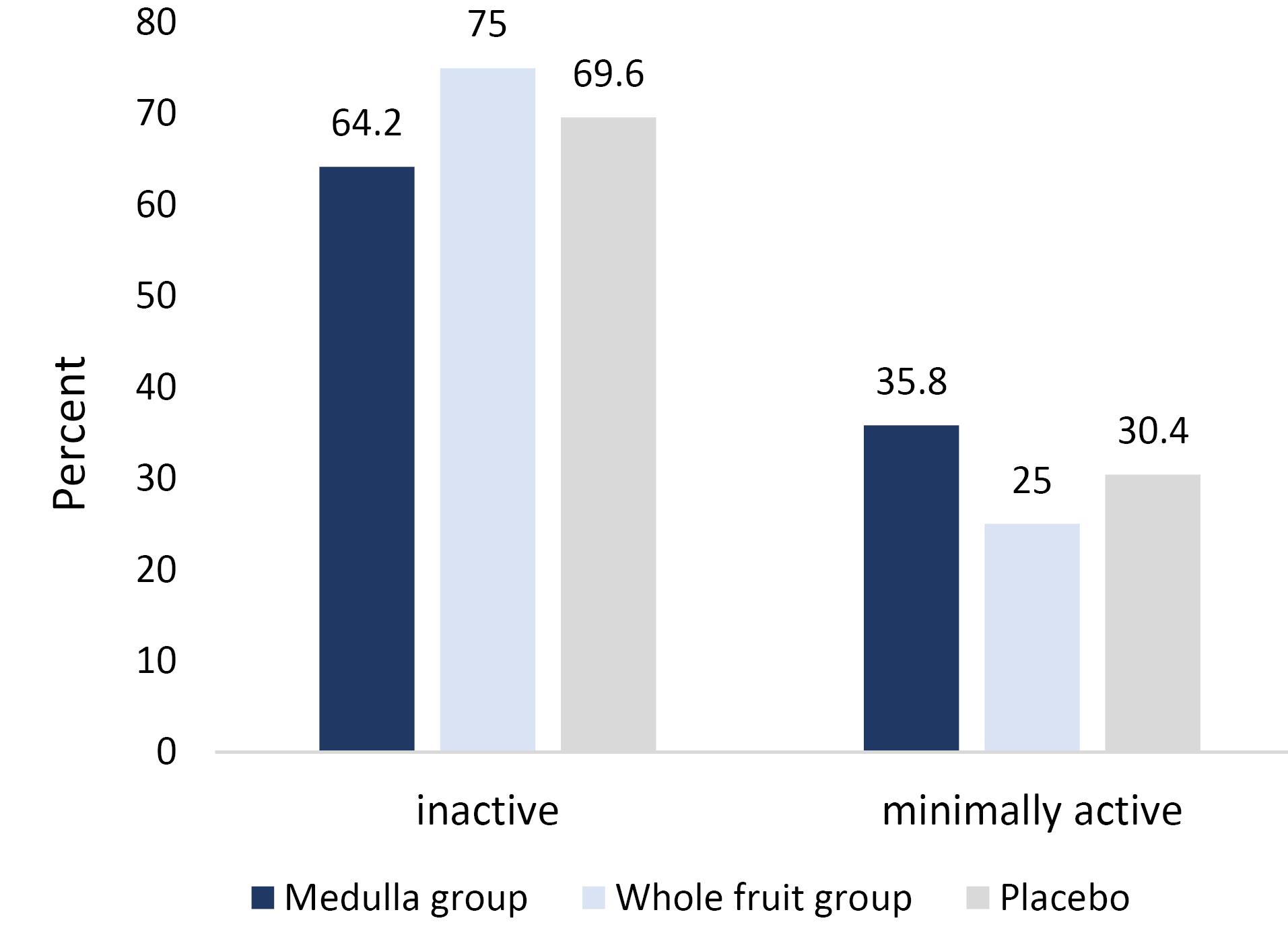
Figure 2.
The Distribution of Physical Activity Level between Different Groups at Baseline
.
The Distribution of Physical Activity Level between Different Groups at Baseline
As illustrated in Figure 3, the mean BMI significantly decreased in the EA medulla (from 32.47 ± 2.92 kg/m2 to 32.29 ± 2.43 kg/m2) and the whole fruit group (from 32.50 ± 2.70 kg/m2 to 32.00 ± 2.87 kg/m2) compared with baseline values, while it increased in the placebo group (from 32.35 ± 2.77 to 32.36 ± 3.27 kg/m2). However, the changes were not significant in the studied groups (P > 0.05).
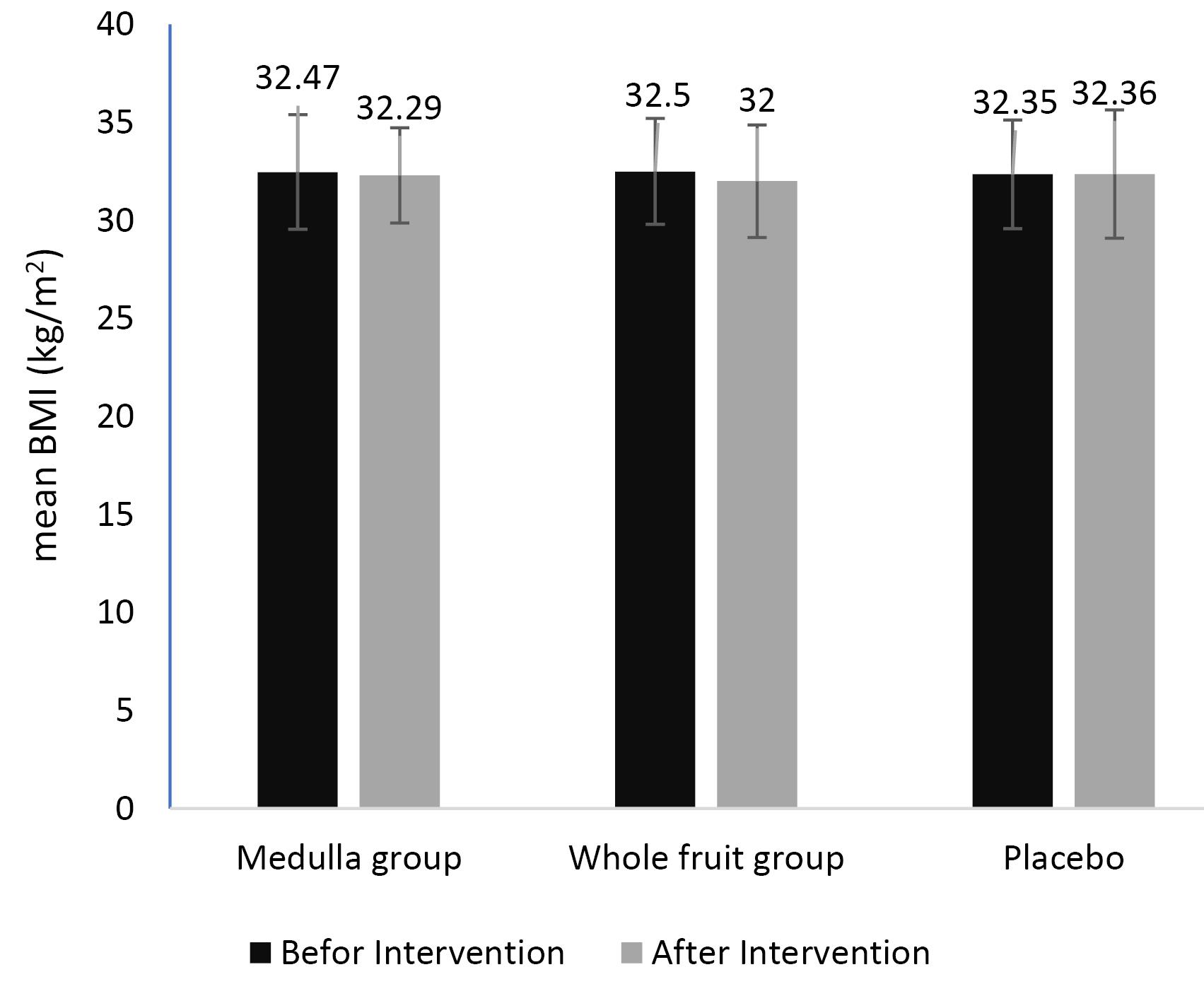
Figure 3.
The Effect of Elaeagnus angustifolia Supplementation on BMI. Note. BMI: Body mass index; SD: Standard deviation; The values are in mean (SD)
.
The Effect of Elaeagnus angustifolia Supplementation on BMI. Note. BMI: Body mass index; SD: Standard deviation; The values are in mean (SD)
As depicted in Table 1, the mean energy intake decreased in EA medulla and whole fruit groups, and it increased in placebo groups. The mean dietary fiber intake increased in the EA groups, while it decreased in the placebo group. Moreover, the results of within-group analyses indicated no significant changes in energy and macronutrient intake after EA medulla, whole fruit, and placebo supplementation compared with baseline values (P > 0.05). In terms of antioxidant micronutrient intake, the result of within-group analysis showed no significant changes in vitamin A, E, C, zinc, and selenium consumption (P > 0.05). Likewise, the result of between-group analyses indicated no significant difference between groups regarding the changes in energy, macro, and micronutrient intake.
Discussion
OA is an inflammation-mediated disease, and studies indicated that metabolic state and inflammatory factors are the main factors of its development and progression.18 Hence, it seems that interventions that focus on weight management could alter the inflammation state in these patients. In this regard, we assessed the effect of EA on anthropometric characteristics and dietary intake of patients. Considering the high fiber and bioactive components,12 we hypothesized that EA could be effective in the weight management of these patients. However, the results indicated no significant differences between the intervention groups and the placebo group regarding the anthropometric characteristics and dietary intakes. To the best of our knowledge, this is the first study that assessed the effect of EA on these parameters. Previously, in an animal model, Karimi et al showed that the consumption of 250 and 500 mg/kg aqueous extract of EA can decrease weight and dietary intake,16 and the weight-reducing effect was higher in groups that received 500 mg/kg extract.16 The effect of EA on weight reduction may be related to its appetite-reducing effect. Tannins are one of the main bioactive components of the EA. Animal studies showed that the high tannins extract can alleviate appetite, prevent the digestion and absorption of nutrients, and consequently reduce weight.19 Alkaloids are another bioactive component in EA that may affect weight by reducing appetite and food intake by acting as an agonist of benzodiazepine receptor.20
EA is also a good source of flavonoids. Quercetin, the main flavonoid found in EA, has a weight-reducing effect. In-vitro studies indicated that quercetin can reduce adipogenesis by activating the mitogen-activated protein kinases (MAPK) signaling path in adipocyte precursor cells.21
The main reason for the non-significant effect of EA on body weight and dietary intake in the present study was the short duration of intervention and follow-up and also the low dose of supplementation. An earlier study on animal models revealed that the effect of EA on weight is dose-dependent, and the weight reduction is higher in higher doses.16 Moreover, although EA had no significant effect on weight and dietary intake in the present study, it may have a positive effect on body composition. Studies indicated that the effect of changes in body composition on clinical symptoms of OA patients is higher than in body weight. No study has assessed the effect of EA on body composition; however considering the flavonoid and fatty acid composition (omega-9 fatty acids) of EA, it can be inferred that EA could have a positive effect on body composition.22,23
This study was the first one that assessed the effect of EA on weight. However, some potential limitations should be considered while interpreting the results. The duration of intervention and follow-up was short. Moreover, the dose of supplementation was low, and the body composition of patients was not assessed in the present study.
Conclusion
The result of the present clinical trial showed that EA has no significant effect on anthropometric characteristics and dietary intake in patients with OA. For more precise results, it is recommended that future studies be conducted with longer follow-up duration and different doses of EA to indicate the best dose of EA with the purpose of weight reduction.
Ethics statement
All included patients signed an informed consent before participation, and the ethics committee of Tabriz University of Medical Sciences approved the protocol of the study (reference number 91171). This trial protocol was registered in the Iranian Registry of Clinical Trials (identifier: IRCT201208241197N13; https://en.irct.ir/trial/408).
Disclosure of funding source
Nutrition Faculty, Tabriz University of Medical Sciences, Tabriz, Iran.
Conflict of interests declaration
The authors declare no conflict of interests.
Acknowledgments
The authors wish to thank the Liver and Gastrointestinal Diseases Research Center, Tabriz University of Medical Sciences for financial support.
References
- Allen KD, Thoma LM, Golightly YM. Epidemiology of osteoarthritis. Osteoarthritis Cartilage 2022; 30(2):184-95. doi: 10.1016/j.joca.2021.04.020 [Crossref] [ Google Scholar]
- Long H, Liu Q, Yin H, Wang K, Diao N, Zhang Y. Prevalence trends of site-specific osteoarthritis from 1990 to 2019: findings from the Global Burden of Disease Study 2019. Arthritis Rheumatol 2022; 74(7):1172-83. doi: 10.1002/art.42089 [Crossref] [ Google Scholar]
- Cooper C, Snow S, McAlindon TE, Kellingray S, Stuart B, Coggon D. Risk factors for the incidence and progression of radiographic knee osteoarthritis. Arthritis & Rheumatism 2000; 43(5):995-1000. doi: 10.1002/1529-0131(200005)43:5<995::aid-anr6>3.0.co;2-1 [Crossref] [ Google Scholar]
- Chen L, Zheng JJY, Li G, Yuan J, Ebert JR, Li H. Pathogenesis and clinical management of obesity-related knee osteoarthritis: impact of mechanical loading. J Orthop Translat 2020; 24:66-75. doi: 10.1016/j.jot.2020.05.001 [Crossref] [ Google Scholar]
- Raud B, Gay C, Guiguet-Auclair C, Bonnin A, Gerbaud L, Pereira B. Level of obesity is directly associated with the clinical and functional consequences of knee osteoarthritis. Sci Rep 2020; 10(1):3601. doi: 10.1038/s41598-020-60587-1 [Crossref] [ Google Scholar]
- Felson DT, Lawrence RC, Hochberg MC, McAlindon T, Dieppe PA, Minor MA. Osteoarthritis: new insights Part 2: treatment approaches. Ann Intern Med 2000; 133(9):726-37. doi: 10.7326/0003-4819-133-9-200011070-00015 [Crossref] [ Google Scholar]
- Silverstein FE, Faich G, Goldstein JL, Simon LS, Pincus T, Whelton A. Gastrointestinal toxicity with celecoxib vs nonsteroidal anti-inflammatory drugs for osteoarthritis and rheumatoid arthritis: the CLASS study: a randomized controlled trial Celecoxib Long-term Arthritis Safety Study. JAMA 2000; 284(10):1247-55. doi: 10.1001/jama.284.10.1247 [Crossref] [ Google Scholar]
- Naqvi R. Osteoarthritis: nutrition and lifestyle intervention. Biol Sci 2023; 3(2):423-38. doi: 10.55006/biolsciences.2023.3205 [Crossref] [ Google Scholar]
- Hashempur MH, Sadrneshin S, Mosavat SH, Ashraf A. Green tea (Camellia sinensis) for patients with knee osteoarthritis: a randomized open-label active-controlled clinical trial. Clin Nutr 2018; 37(1):85-90. doi: 10.1016/j.clnu.2016.12.004 [Crossref] [ Google Scholar]
- Rondanelli M, Riva A, Allegrini P, Faliva MA, Naso M, Peroni G. The use of a new food-grade lecithin formulation of highly standardized ginger (Zingiber officinale) and Acmella oleracea extracts for the treatment of pain and inflammation in a group of subjects with moderate knee osteoarthritis. J Pain Res 2020; 13:761-70. doi: 10.2147/jpr.s214488 [Crossref] [ Google Scholar]
- Nikniaz Z, Ostadrahimi A, Mahdavi R, Ebrahimi AA, Nikniaz L. Effects of Elaeagnus angustifolia L supplementation on serum levels of inflammatory cytokines and matrix metalloproteinases in females with knee osteoarthritis. Complement Ther Med 2014; 22(5):864-9. doi: 10.1016/j.ctim.2014.07.004 [Crossref] [ Google Scholar]
- Hamidpour R, Hamidpour S, Hamidpour M, Shahlari M, Sohraby M, Shahlari N. Russian olive (Elaeagnus angustifolia L): from a variety of traditional medicinal applications to its novel roles as active antioxidant, anti-inflammatory, anti-mutagenic and analgesic agent. J Tradit Complement Med 2017; 7(1):24-9. doi: 10.1016/j.jtcme.2015.09.004 [Crossref] [ Google Scholar]
- Farzaei MH, Bahramsoltani R, Abbasabadi Z, Rahimi R. A comprehensive review on phytochemical and pharmacological aspects of Elaeagnus angustifolia L. J Pharm Pharmacol 2015; 67(11):1467-80. doi: 10.1111/jphp.12442 [Crossref] [ Google Scholar]
- Nikniaz Z, Mahdavi R, Ostadrahimi A, Ebrahimi A, Nikniaz L, Vatankhah A. Effects of Elaeagnus angustifolia L powder supplementation on serum total antioxidant capacity and malondialdehyde levels in females with knee osteoarthritis. J Herb Med 2015; 5(4):177-83. doi: 10.1016/j.hermed.2015.09.003 [Crossref] [ Google Scholar]
- Nikniaz Z, Mahdavi R, Nikniaz L, Ebrahimi A, Ostadrahimi A. Effects of Elaeagnus angustifolia L on lipid profile and atherogenic indices in obese females: a randomized controlled clinical trial. J Diet Suppl 2016; 13(6):595-606. doi: 10.3109/19390211.2016.1150933 [Crossref] [ Google Scholar]
- Karimi G, Khoie A, Hosseinzadeh H, Shojaie S. Subacute toxicity of Elaeagnus angustifolia fruit seeds in rats. J Med Plants 2003;2(8):47-54. [Persian].
- Vasheghani-Farahani A, Tahmasbi M, Asheri H, Ashraf H, Nedjat S, Kordi R. The Persian, last 7-day, long form of the international physical activity questionnaire: translation and validation study. Asian J Sports Med 2011; 2(2):106-16. doi: 10.5812/asjsm.34781 [Crossref] [ Google Scholar]
- Jiang H, Pu Y, Li ZH, Liu W, Deng Y, Liang R. Adiponectin, may be a potential protective factor for obesity-related osteoarthritis. Diabetes Metab Syndr Obes 2022; 15:1305-19. doi: 10.2147/dmso.s359330 [Crossref] [ Google Scholar]
- Lei F, Zhang XN, Wang W, Xing DM, Xie WD, Su H. Evidence of anti-obesity effects of the pomegranate leaf extract in high-fat diet induced obese mice. Int J Obes (Lond) 2007; 31(6):1023-9. doi: 10.1038/sj.ijo.0803502 [Crossref] [ Google Scholar]
- Cooper SJ. Beta-carbolines characterized as benzodiazepine receptor agonists and inverse agonists produce bi-directional changes in palatable food consumption. Brain Res Bull 1986; 17(5):627-37. doi: 10.1016/0361-9230(86)90194-2 [Crossref] [ Google Scholar]
- Hong SY, Ha AW, Kim W. Effects of quercetin on cell differentiation and adipogenesis in 3T3-L1 adipocytes. Nutr Res Pract 2021; 15(4):444-55. doi: 10.4162/nrp.2021.15.4.444 [Crossref] [ Google Scholar]
- Amiri Tehranizadeh Z, Baratian A, Hosseinzadeh H. Russian olive (Elaeagnus angustifolia) as a herbal healer. Bioimpacts 2016; 6(3):155-67. doi: 10.15171/bi.2016.22 [Crossref] [ Google Scholar]
- Sato S, Mukai Y. Modulation of chronic inflammation by quercetin: the beneficial effects on obesity. J Inflamm Res 2020; 13:421-31. doi: 10.2147/jir.s228361 [Crossref] [ Google Scholar]